
Passing grade is 70%. HW is 10%, quiz 10%, final 80%.
Train operators to provide safe water at a reasonable cost.
The SDWA was passed by the Senate in mid 1973, by the House on November 19, 1974 and signed into law on December 16, 1974 by President Ford.
The Congress had been debating national drinking water standards for several years. The need for national requirements became evident when it was found that out of 446 water systems studied only 60 met existing federal standards for bacteria content and testing frequency. Moreover, the existing standards applied only to contaminants which could cause communicable diseases and not to chemical toxins. Concurrently, organic chemicals were discovered in New Orleans' drinking water. In 1975 a follow up study found organic contamination in 80 cities' water.
The SDWA directed EPA to set up national primary drinking water regulations applicable to public water systems throughout the country.
The primary regulations concern matters directly affecting the health of the consumer.
In addition, secondary regulations dealing with the aesthetic qualities of drinking water (color, taste, odor, etc.) were created as guidelines.
In New York state, Part 5 of the sanitary code directly applies to all water supplies (as defined) and all operators. Have it handy. Use it.
Important note: For the most part, this manual avoids direct quotation of the sanitary (or any other) code. The codes change. Use the current version. Use the code which applies to your jurisdiction. Do not use this manual as a substitute for or summary of the relevant codes. Get your copy of the code now.
Part 5 of the New York State Sanitary Code was enacted by New York in order to provide a legal framework for the state to enforce the provisions of the SDWA. In some cases Part 5 is more stringent than the SDWA. As the SDWA, or the regulations promulgated under SDWA by the US EPA, is modified and expanded, Part 5 is updated by New York, so that the state may continue to comply with and enforce the provisions of the SDWA.
Appendices A and B of Part 5 are also known as Ten States and Rural Water Supply. Ten States has design information for water supplies. Rural Water Supply has design, construction, and operation information for small water supplies.
As N
SOCs, POCs, UOCs
A knowledge of addition, subtraction, multiplication, division, and basic algebra is necessary. The algebra consists of rearranging formulas or solving for unknowns.
Describe what parameter the numerical value represents (lbs, feet, inches, etc.). Give dimensions to values. Include units on all numerical values. Include units in calculations and intermediate values. Units can be multiplied (ft x ft = sq. ft or ft2) and divided. Dividing a unit by itself yields 1 (the unit cancels itself - 4 sq. ft / 2 ft = 2 feet). Check units in answer to ensure that the calculation has been done properly.
Metric units are often composed of a prefix which determines how many and a root which explains what is being measured.
|
Prefix |
abbreviation |
meaning |
Root |
abbreviation |
meaning |
|---|---|---|---|---|---|
|
Mega |
M |
million |
gram |
g |
mass |
|
Kilo |
K |
thousand |
litre |
L or l |
volume |
|
milli |
m |
1/1000 |
volt |
V |
electrical potential |
|
micro |
μ or u or mc |
one millionth |
meters |
m |
length |
| Scale | Freezing | Boiling | Groundwater | Surface water |
|---|---|---|---|---|
| Celsius | 0 | 100 | 10 | 0-24 |
| Fahrenheit | 32 | 212 | 50 | 32-75 |
F=Cx1.8+32
C=(F-32)/1.8
In equations, temperature is usually represented by 'T'. Do not confuse this with time, which is usually represented by 't'.
Linear distance; one dimension.
| unit | symbol | example |
|---|---|---|
| inches | " | 1" diameter pipe |
| feet | ' | 60' high tank |
Two dimensions
"square" units. E.g. square inches, sq. ft.
Rectangular area is calculated by multiplying the length by the width
Triangular area = 1/2 x base x height
In equations, area is usually represented by 'A'.

The radius, r, is the length from the center of a circle to the edge of the circle. The diameter, d, is the length across the circle through the center. The diameter is thus twice the radius. The circumference is the length of the circle perimeter. The circumference is equal to πd or 2πr.
Π (pi or π) is a constant equal to about 3.14. Pi is often found in circle formulas.
The area of a circle is equal to πr2. Substituting d/2 for r, A=πd2/4. Substituting 3.14 for π, A=0.785d2.
Volumes are measured in three dimensions of length. Often the units will be "cubic" such as cubic feet (ft3) or cubic inches (cu. in., in3). Other volume units just describe a certain space: gallons, liters.
Volume = length x width x height. Since Area = length x width, volumes can also be expressed as area x height. This is very useful for shapes that do not have a rectangular cross section, such as cylinders.
Volume shape names
| Name | Shape | Example |
|---|---|---|
| cuboid, box | box, cube | rectangular tanks, buildings |
| cylinder | circular cross section | pipe, can, bucket, tank, well |
| sphere | ball | some water towers |
In equations, Volume is usually represented by 'V'. Do not confuse this with velocity which is usually represented as 'v'.
Velocity is the measure of distance over time. Technically, velocity also has direction, unlike speed, which is just how fast you are going. Examples are:
| Miles per hour | mph |
| inches per day | in/day |
| feet per second | fps |
The speed of the water moving through a pipe is important because it affects settling, clogging, scaling, erosion, corrosion, mixing, friction, energy loss, and the forces on the pipe and joints.
In equations, velocity is usually represented as 'v'. Do not confuse this with Volume, which is usually represented by 'V'.
Flow is the measure of volume over time. Examples are:
| Gallons per minute | gpm |
| Gallons per day | GPD |
| cubic feet per second | cfs |
In equations, flow is usually represented by 'Q' for quantity.
Mass is the measure of matter. Weight is a measure of how much forces affect matter. Primarily, this means the force of gravity. When the gravity at the surface of the earth attracts a gallon of water, it pulls with 8.34 pounds. As long as you are near the surface of the earth, weight and mass are interchangeble (at least as far a the Grade C operator is concerned). Weight can be measured in pounds, ounces, grams, kilograms, tons, etc.
Pressure is force (or weight) divided by area. Some pressure units reflect pressure's relation to force and area: pounds per square inch. Other pressure units are based on commonly found pressures: atmosphere or barr, millibarr. Still other pressure units are based on columns of fluids: inches of mercury, feet of water. The pressure in any fluid is directly proportional to the depth of the fluid and the density (or weight) of the fluid.
In equations, pressure is usually represented by 'P'.
The strength of a solution is often referred to as its concentration. More concentrated solutions have more "stuff" dissolved in them. "Weaker" solutions have less "stuff" dissolved.
Concentration is normally expressed as the mass (or weight) of the dissolved substance divided by the volume of the total solution. For example, 3 milligrams per liter of chlorine (in water). Concentrations are also expressed as "per cents", meaning what percent of the total solution is the dissolved "stuff". E.g. 5% chlorine (which is therefore 95 percent water). Percents are usually expressed as a weight to weight ratio rather than weight to volume, as in the first example.
In equations, concentration is often represented by 'C'.
Units which measure the same property can be readily converted from one to another. Inches and feet both measure distance. There are 12 inches in a foot. To obtain feet from inches, divide by 12.
A convenient way to remember whether to divide or multiply is to set up the conversion as a fraction with one unit on the top and an equal quantity on the bottom: 12 inches/foot. Because 12 inches = one foot, the fraction's value is one. You may then multiply this fraction by feet to obtain inches since any quantity multiplied by one will yield the same quantity: 3 feet x (12 inches/foot) = 36 inches.
It is also useful to remember which unit is bigger. Since feet are bigger (longer) than inches, you will always expect that a pipe will have more inches than feet. A 6 foot pipe is 72 inches. Knowing what answer to expect makes picking the right conversion factor easier.
For units with prefixes (like milligrams) you can substitute the prefix meaning for the prefix. 5 milligrams becomes 5 (1/1000) grams or 5/1000 grams or .005 grams.
Conversion factors:
12 inches = 1 foot
7.48 gallons = 1 cubic foot
1440 minutes = 1 day
1000000 gallons = 1 million gallons
Physical substances have many properties. Often there are constant relations between these properties. A given volume of water usually has a fixed weight. This constant (normally) relation allows us to express a quantity of water in weight or in volume and to convert from one method of expression to another.
Conversion factors
1 gallon of water = 8.34 pounds
1 vertical foot of water = .433 pounds/square inch
The result of a calculation can be no more precise than the least precise input. Round off answers as necessary.
6.2 feet x 5.1 feet = 31.62 ft2 which should be rounded to 32 ft2 since the lengths have only 2 significant figures.
| 11.078 | 5 significant figures |
| 21,000 | 2 significant figures. The zeros just take up space. |
| 0.00325 | 3 significant figures |
| 87.62 | 4 significant figures. |
X*1=X, X*0=0, X+0=X, X/X=1
Use the above identities to simplify equations.
You may multiply or divide both sides of an equation by the same non-zero value. Know how to "solve for X" by rearranging equations. 'X' is often replaced by other standard letters as appropriate. See Typical units used in water systems, above.
You may always substitute the left side of the equation for the right side of the equation (because that's what EQUAL means).
The arithmetic mean is what most people call the average. It is found by adding the values and dividing the result by the number of values added.
If you pumped 400 gallons one day, 500 gallons the next, and 390 gallons on the day after that, then the average gallons per day pumped is
=(Q1 + Q2 + Q3) / n
=(400 gallons + 500 gallons + 390 gallons) / (3 days)
=430 gallons per day
Some phenomena do not obey a linear relationship. For example, bacterial growth is often exponential. Each time the bacteria reproduce, they double. So after each time period, there's twice as many bacteria. If you are growing several batches of bacteria in order to count how many you have in your water, and you let one batch sit a bit too long, that batch will double in number and make your average appear higher than it should.
The geometric mean is used to find a truer average for bacterial growth and for other phenomena which follow exponential or geometric functions. It is found by multiplying the values and taking the nth root of the result, where n is the number of values multiplied. You can also take the antilogarithm of the arithmetic mean of the logarithms of the values.
=(Q1 * Q2 * Q3)^(1/n) or
=exp((ln(Q1) + ln(Q2) + ln(Q3)) / n)
You can use base 10 logs if you like; the base doesn't matter.
If you got results of 10 colonies, 50 colonies, and 100 colonies from your standard plate count tests, the arithmetic mean would be 80 colonies. The geometric mean would be
(10 * 50 * 100)^(1/3) = about 37 colonies.
The units were left out on the left hand side for clarity, don't do that at home.
Note to calculator users: Multiplying large numbers together can overflow you calculator. Most regular calculators have a limit of about 99,999,999 or 10^8. Most scientific calculators have a limit of about 10^99. Most computer software has a limit of 10^308.
Precision is the exactness of a number. Accuracy is the "rightness" of the answer. 49783 is more precise than 50000. 50000 is more accurate if the actual value is 51225. A quartz watch may be very precise in its display of the time, but is only as accurate as the time it was set with. Be careful calibrating instruments. Calculators often are capable of displaying 8 digits. Calculated results may contain many digits, for example 12.56398 feet. Not all of the digits in an answer will be meaningful. 0.00098 feet is thinner than most hair. The result of a calculation can be no more accurate than the numbers originally entered in. Such numbers come from the real world; from pressure gauges, from tape measures, from scales. Just as you can not measure the width of a hair with a tape measure, any calculated result must match the precision of the device that generated the original numbers. See significant figures, above
Make sure that the units on one side of an equation equal those on the other side. Know which units can be converted into which others. Look at the different units in the question and in the answer for clues as to how to proceed. For example if you are given gallons and minutes, and asked to figure out gallons per minute, you may want to be dividing the gallons by the minutes.
Only add like units. Never add two numbers with different units. Always convert one number to the units of the other. When multiplying or dividing, always multiply or divide the units. Look for ways to simplify the units. Cancel units which appear on both the top and bottom of a fraction.
Many common verbs and prepositions correspond to mathematical operations. Also look at units to give clues as to types of information.
Multiply words: at, by. 12 apples AT $.35 each. 4 BY 4
Divide words: per, in, of. miles PER hour; 4 out OF 5 dentists
Add words: and, plus
Subtract words: difference, from, minus, more, less
Distance words: length, width, depth, height, far
Circumference words: girth, around
Area words: circular, round, square, rectangular, cross section
Volume words: tank, gallons
Time words: how long, duration, when
Mass/weight words: heavy, amount
Concentration words: strength
There are always several ways to solve a problem. Notice the three equations for the area of a circle (above). Find a formula, or approach, that makes sense to you and don't worry about the others. Don't be upset if your system is different from mine or your neighbors'.

Graphs are useful for showing the relationships between parameters (variables, sets of numbers, things). Often a graph will convey information (trends, correspondences) that a list of numbers will not. Graphs can be very useful when explaining technical details to the public or to management.
The axes of a graph represent the variable being shown. They should be labeled with descriptions and units. The vertical axis might show gallons pumped, the horizontal might show months:
A knowledge of how water behaves, both in nature (in the ground and in streams and lakes) and in plumbing (pipes, pumps, filters, etc.) is very useful when installing modifications or troubleshooting problems in water systems.
Water weighs 8.34 pounds per gallon.
Water is incompressible. The volume of water does not change when the pressure changes.
Pressure is a force acting across an area. The weight of water presses against the water's container.
Pressure is directly related to depth. The pressure in the water at a certain depth is related to the weight of the water above it. A one foot cube of water weighs 62.4 pounds (8.34 pounds/gallon * 7.48 gallons/ft3). That cube sits on a one foot square base or on 144 square inches. The pressure on the bottom is therefore equal to 62.4 pounds/144 square inches or 0.433 psi (pounds per square inch).
0.433 psi corresponds to the pressure at the bottom of 1 foot of water. In the water industry it is common to move water up or down. The pressures required to create this movement and the pressures created by heights of water are often referred to by the height of the water. This is known as the head and is commonly measured in feet. Pumps are often rated by how much water (volume per time, gallons per minute) they can pump up a certain distance (head or feet).
For a 300 foot deep well at a home, the well pump should produce 5 gallons per minute against 300 feet of head. Such a pump could pump water even when the water level in the well was near the bottom. In practice, the pump also must pump against the pressure in the hydropneumatic (also known as the pressure) tank.
Water pressure (as in all fluids) acts in all directions.
Pressure against an area produces force. How much force does the end cap of a 12 inch water main restrain if the pressure in the water main is 65 psi? The area of the cap is πr2. The radius (r) is 6 inches. The area is 113 square inches. The force is P*A or 65 psi * 113 square inches. The force is over 7 thousand pounds.
The atmosphere resting on one square inch of land (or anything else) at sea level weighs 14.7 pounds. The atmospheric pressure is therefor 14.7 psi. Most gauges read zero at atmospheric pressure even though the absolute pressure is 14.7 psi. You may see units like psia and psig (pounds per square inch absolute and psi gauge) used to clarify what is being measured.
A total vacuum is 0 psi. A pump which can draw a total vacuum can pull water up 34 feet at sea level. The pump is not really pulling anything. The atmospheric pressure on the rest of the water is pushing it up.
Water flows from areas of high pressure to areas of low pressure.
 Q=AV or Flow = Area times Velocity.
Q=AV or Flow = Area times Velocity.
This is related to conservation of mass. All the water that flows in a pipe is flowing at the same speed. If water at some point were flowing faster, it would have to compress against the water in front of it and it would leave a vacuum between it and the water behind it. It can't do this because water is incompressible.
Similarly, if a pipe is full, then for any water that flows in to the pipe, an equal amount must flow out of the pipe. This also applies to joints and tees. The volume of water entering a tee is equal to the volume leaving the other two legs.
As water flows, it rubs against the walls of the pipe (or whatever it is flowing in) containing it. This friction causes a drop in pressure, since continuity prevents a drop in velocity. Pressure drops due to friction only happen when the water is moving.
In filters the water rubs against the walls of the filter and the media of the filter. Filters usually have very high pressure drops. Reduction in pressure is also known as head loss.
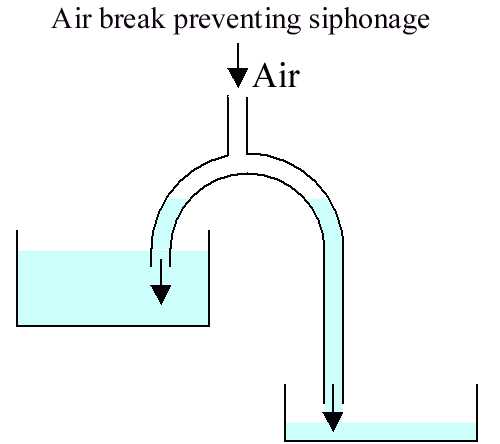
Because the inlet pressure is higher than the outlet pressure, water will flow. Remember that pressure is independent of path - pressure is determined solely by depth. The pressure in the part of the siphon above the level of the upper reservoir will be less than the atmospheric pressure.
Siphons must be primed.
Anti siphon valves work by opening the low pressure zone of the siphon to the air. Water can be pushed through the siphon but won't flow by itself nor can water be "pulled" through.

Flowing water is powerful. Literally. Water powered the mills of the industrial revolution and continues to generate electricity today. Mostly, we use power to move water rather than moving water to generate power. The power contained in flowing water is equal to the flow times the head (pressure).
How much power does it take to pump 5 gallons per minute up 100 feet? This is a typical condition in a drilled well. 5 gpm * 100 ft = 500 gpm ft. To convert gpm ft to horsepower, divide by 3960. To convert horsepower to watts multiply by 700. For fun, check to see if 1 horsepower = 550 lb ft per second.

Expensive to extract.
Describes how water moves from one place to another.
| Evaporation | Vaporization of water from the ocean, streams, lakes, ground. May include transpiration from plants. Liquid water becomes a gas (and enters the atmosphere) |
| Precipitation | Rain, snow. Atmospheric water becomes liquid or solid and hits the earth's surface |
| Runoff | Precipitation which flows along the surface rather than soaking in |
| Infiltration | Precipitation which seeps into the ground |
Water is constantly moving from one of the above places to another. Among these movements are Rain, Evaporation, Transpiration, Runoff, Flow, etc.
Movement of water is important to move water to your well or reservoir.
In addition to moving water from place to place, the hydrologic cycle also purifies water. Evaporation distills the water. Subsurface flow can filter the water.
Surface supplies are preferred for large users (cities) because of the large volume of available water and the ease of access to the water. Small systems rarely use surface water.
The water quality of reservoirs is quite variable. Even though a stream may run through a reservoir, that flow is rarely sufficient to keep the reservoir well mixed. Cold water is very slightly denser than warm water. Therefore, cold water sinks to the bottom of the reservoir. In the warm seasons, the surface of the water is exposed to sunlight and warm air which warm the upper layers. The lower layers are in contact with the ground, which is cooler (10 degrees C, in most places). Thus the upper and lower layers do not mix. This layering of water is called stratification. In the winter, the upper layers are cooled by the air until they are colder than the lower water. Then the reservoir "turns over" as the upper layers sink. Reservoir turnover can greatly effect water quality if the two layers have different qualities. Often, the lower layer will not have much oxygen but will contain dead algae which has sunk from the upper sun lit layers.
Why is stratification important?
Preventing mixing among layers allows the water quality of the layers to differ. Lower layers are not exposed to the atmosphere so get no oxygen. Oxygen changes the properties of many chemicals. Compare hydrogen sulfide with sulfate, or iron with rust.
Reservoir (and lake) terms: Stratification, turn over, thermocline, hypolimnion, hyperlimnion.
Intake structures should have inlets at various levels to allow use of top, middle, or lower layers of water.
Surface water quality is dependent upon the characteristics of the watershed which collects it. Surface water quality changes much more quickly and varies more widely than ground water. Surface water is prone to contamination from actions taken in the watershed and from accidents. Surface water is more likely to have algae, which can cause taste and odor problems. Algae or other vegetation can cause color problems.
Surface water sources can be quickly affected by accidents such as chemical spills and transportation accidents.
The water-saturated subsurface geologic formations which are now or may be developed to supply water in usable quantities to public or individual wells.
Any geologic formation containing water, especially one that supplies water for wells, springs, etc.
Unconfined (water table) aquifer
Water can be stored in the spaces between the grains of soil. The more uniform the particle size, the more space is available for water. If different particle sizes are present, the smaller particles can fit between the larger ones, taking up space that water could have been stored in. The volume of space in a volume of soil is the porosity. Soils with uniform particle size have greater porosity than soils with many particle sizes.
The larger the soil particles, the larger the spaces between them are. Water can move faster through larger spaces because there is less contact with the channel walls and therefore less friction.
Water soluble rocks such as limestones and other calcium carbonate rocks are dissolved by as water flows through or along them. Large channels capable of holding and transporting large quantities of water can be formed in these types of rocks. If the solution channels become too big, the area may be known for sinkholes and may be referred to as karst.
Water seeping into the ground joins an aquifer. The area of the ground surface on which water seeps into a particular aquifer is that aquifer's recharge area. Any water (or contamination) landing on the recharge area will enter the aquifer (and later be pumped into water systems which get their water from that aquifer). Water may take years or millennia from the time that it falls as rain to the time it is pumped out of the aquifer.
Confined Aquifer
Ground water which is overlain by impermeable deposits. Often, this water will be under pressure.
Ground water not confined. The upper surface of this type of aquifer is the water table.
Because ground water travels for long periods of time through rock and soil, its quality varies little and slowly. Groundwater is usually well filtered but may be very hard because it has dissolved minerals from the rocks and soil. Ground water is also susceptible to contamination, however, the area of recharge is much harder to delineate.
Can be surface or ground water depending on development. Best to eliminate surface water with properly constructed enclosures.
AWWA specification A100-90
Dug wells are shallow (20-30 feet) with little penetration of the water table. Their output is usually variable because of water table fluctuations. Water quality may also be variable. Contamination may be caused by surface water seepage. The well curbing should extend above the ground surface at least one foot. There should be a watertight cover, penetrated only by the water withdrawal pipe and or vent, to prevent contamination.
A drilled well has the following parts.
Rotary.
Rotary bit grinds rock. Water or air drilling fluid cools bit and carries spoil back to surface. The drilling fluid is often called 'mud'.
Most common well drilling method. Quick. Capable of great depths. Rotary bit tends to force rock dust into water seams.
Percussion.
Heavy bit is dropped onto rock. Bit is exchanged for bucket which draws up rock fragments.
Slow. Only capable of moderate depths (~200'). Tends to suck crushed rock out of seams.
The casing has several jobs. In soft or loose soils and rocks the casing prevents the sides of the drill hole from collapsing into the well. The casing also helps keep contamination out of the well by sealing off the top portion of the well from surface water and upper aquifers. This function is done in combination with the grout.
Grout is used to fill the space between the casing and the drill hole. Grout prevents surface water from gaining access, prevents mixing of different aquifers along the outside of the casing. Grout also supports the casing.
A suitable material for grouting should not shrink upon drying ( and thus defeat the purpose by leaving a gap between the grout and the rock or the casing). Better materials expand to fill any cracks or voids. Bentonite clay, neat cement and concrete are used for grouting.
Grout should be placed from the bottom up in one continuous operation. A tremmie pipe is used to ensure that grout does not get stuck midway and leave a void below.
The screen prevents loose sandy deposits from collapsing into the bore hole. Screens can be made by winding steel or by cutting slots in casing.
The screen slots must be sized to allow enough water into the well without creating excessive water velocity at the slots. Changing the velocity changes the pressure in the water (see Bernoulli) altering the chemical balance and depositing minerals in the screen.
Screens may be cleaned chemically by use of acids (usually muriatic or acetic). Acetic is less likely to also dissolve the screen. Mechanical means such as water jets are also available.
In very fine grained formations, the screen may be gravel packed to prevent fines from entering the well. The sand is trapped in the gravel surrounding the well screen.
The cap should provide a sanitary seal to prevent any contaminants from entering the well. Insist on an air tight seal (except for the vent!).
If the drop pipe supports a submersible pump, brackets called torque arrestors will often be installed along the drop pipe to transmit torque from the pump to the casing, rather than allowing the drop pipe to absorb the torque.
Vents should be free from flooding. Generally a U tube with a screen is installed. The screen prevents insects or animals from getting into the well.
This process injectes carbon dioxide into the aquifer resulting in the formation of carbonic acid, a mild acid. The acid will dissolve some minerals from the aquifer and well screen and may increase well yield. The cost is approximately $1800 (2002).
Surging is used to remove soft clogging material from the well, such as, iron bacteria and soft rock. A cable tool well drilling rig with a packer on the cable will be used to surge the well.The packer is continuously rasied and lowered creating a surging action in the well. At the end of the process the well is bailed to remove any debris from the bottom. The cost is approximately $1800 (2002).
The hydrofracturing process (also hydrofrac, hydrofrack, and hydrofract) can be used to increase the yield of the well, the efectiveness of this process depends on the characteristics of the aquifer. The process uses high pressure (3000 psi) water injection to dislodge soft rock from fractures. In the single packer method a packer is placed at approximately 100 feet and the entire bore hole below the packer is pressurized. The packer is then lowered 100 feet and the process is repeated. This continues for the remainder of the bore hole. The cost is approximately $2500 (2002). In the double packer method two packers about 60 feet apart are used and the high pressure water applied between the packers. The entire well will be hydrofractured using 60 foot intervals. The cost is approximately $6000 (2002). About 1500 gallons of water may be injected into the well during the process.
Wells are a conduit connecting different aquifers with the surface of the ground. In addition to allowing the transfer of water from the aquifer to the surface (where we use it beneficially), wells can allow transfer of contamination from the surface (or a contaminated aquifer) to a clean aquifer.
Unused wells should be properly abandoned to prevent the possibility of contaminating the groundwater. Proper abandoment procedures vary with the geology in the area. Appendix G of AWWA standard A100 lists various procedures.
The area where a pumping well has lowered the groundwater table or lowered the head in a confined aquifer.

The area from which a pumping well draws water. If you spilled water on the ground it will enter the well if you are in the zone of influence. If the groundwater is moving, the cone of depression and the zone of influence will not coincide.
The difference between the static water level and the pumping level.
Short term well tests are misleading because they measure stored water. This water may be perched water or the water that was in the cone of depression. A sustained (often 72 hours) yield test gives a value closer to the long term well yield.
Used to determine the most efficient well pumping rate.
Discharge water away from the well so that no recirculation or recharge occurs.
Pure water is rarely found in nature. Rainwater is actually very pure, except for the sulfate and nitrates the rain absorbs from the combustion products of the industrial revolution. This phenomenon is also refered to as "acid rain". The quality of the water from your wells or reservoirs is influenced by the path the water takes from rain drop to your intake pump. Here are some of the contaminants you may find in your source water:
Carbonates from limestones
sulfur from gypsum, pyrite, etc.
iron, manganese, zinc
Nitrates from fertilizers or sewage
Chlorides from home softeners or road salting
Freons from refridgerants in air conditioners and refridgerators, or from industrial cleaners.
Aromatic hydrocarbons from fuel oils
Chlorinated hydrocarbons from solvents and cleaners
Some organic chemicals are only slightly soluble in water. The undissolved part may be heavier than water and sink to the bottom of the aquifer. It may be lighter and float. It may prefer to stick to the soil, and therefor move more slowly than the groundwater.
See also the Importance of Water section
One celled living creatures. Larger and more sophisticated than bacteria.
One celled living creatures.
Very small parasites. Viruses can only reproduce by taking over the cells of a host organism.
In accordance with the maxim "An ounce of prevention ..." protecting the well and the aquifer from becoming polluted is often more efficient than removing the pollutants at the treatment plant.
The following methods can be used to estimate the zone of influence of a well.
Be careful using chemicals onsite.
Owning all the land in the watershed gives you control over the practices that take place there. Ownership of the entire watershed is impractical, but ownership of critical areas is advisable.
Easements convey specific abilities to restrict actions in the watershed. Easements are easier to obtain than ownership.
In most cases, the water for a well or reservoir comes from far away. Way beyond the area practical for ownership or private easements. Way beyond the political boundaries of the local municipality served by the water system. The New York State Public Health Law has a provision to allow local water supplies to gain state protection for their watersheds by incorporating regulations into the New York State Code of Rules and Regulations. In this manner, a water supply can gain control over areas that are in other towns or counties.
The source water quality must meet the requirements of Part 5, which are currently contained in the tables in section 5-1.52. The source water quantity requirements are contained in the design manuals. Briefly, the water sources must be able to supply the maximum day demand, which is the amount of water used on the day when the most water is used. The maximum day demand often occurs in the summer on a holiday. Usually, the maximum day demand is about twice the average day demand. The sources must be able to produce the average daily demand without utilizing the largest source. Therefore at least two sources are required. If one well is broken, dry, contaminated, or being maintained, the system will still be able to run. The design standards limit well use to 16 hours per day.
| Vector | Disease |
|---|---|
| Bacteria | |
| Vibrio cholera | Cholera |
| Shigella sonnei | Bacterial Dysentery |
| Salmonella typhi | Typhoid Fever |
| Salmonella paratyphi | Paratyphoid Fever |
| Salmonella enteritidis | Gastroenteritis |
| Legionella pneumophila | Legionnaire's disease |
| Yersinia enterocolitica | |
| Campylobacter jejuni | |
| Viruses | |
| Numerous forms isolated from human feces |
Infectious Hepatitis Myocarditis (heart disease) Poliomyelitis (suspected) Viral Gastroenteritis Viral Diarrhea |
| Parasitic Protozoans | |
| Amoeba histolytica (Entamoeba) | Amoebic Dysentery |
| Giardia Lamblia | Giardiasis |
| Cryptosporidium Parvum | Cryptosporidiosis |
| Cyclospora |
Most waterborne diseases have similar symptoms, regardless of the particular agent or vector of transmission. We divide them by their biological distinctions because of their different removal considerations.
Bacterial diseases were among the first to be discovered and, thus, the most is known about bacteria, including types, symptoms, treatments, and how they work. Bacteria are one celled "animals" which can live and reproduce, sometimes in humans. Some bacteria directly cause infection, others produce byproducts which are toxic. Many bacterial diseases can be treated with antibiotics.
These are the smallest biological agents. They require a host organism in order to reproduce. They do not eat or move by themselves. For these reasons they are difficult to test for. Most viral diseases can not be treated medically. Some of their symptoms (such as dehydration) can be treated.
Protozoans are larger and more sophisticated than bacteria. Some form a cyst (or oocyst) with a hard protective coat. The protective coat makes them difficult to kill with chlorine. Giardia cysts range from 6-10 micrometers. Cryptosporidium cysts are 2-6 micrometers. Cryptosporidium is present in roughly 75% of surface waters tested in the United States. Cyclospora is twice as big as cryptosporidium.
Although there has been a sharp decline in the incidence of waterborne diseases in the United States in the last century, there has been a leveling off of the decrease and even an increase in outbreaks since the 1950's. The major cause of outbreaks in public water supplies is through contamination of the distribution system, but contamination of the source or a breakdown in disinfection, though responsible for fewer outbreaks, results in a far greater number of illnesses. Cycles are popular in nature. Just as water evaporates from the sea to the air, rains into the rivers, which flow back to the sea, so too do diseases cycle. A typical waterborne disease cycle may start with a contaminated drinking water source. Customers drink the water and become infected, their waste (feces) then recontaminate the well. One goal of the water operator is to break this cycle. Every link in the cycle is an opportunity. Protecting the well and distribution system from sewage and other contaminants; disinfecting the water to eliminate pathogens, and preventing users from drinking contaminated water are possible strategies.
The portal of entry is the route by which a pathogen obtains access to the body. The skin protects us from all manner of hazards, in addition to keeping our insides in. Some organisms attack the skin itself (worms, mosquito borne diseases), most exploit holes in the skin (the mouth, ears, nose, eyes, or wounds).
Legionnaire's disease spends part of its life cycle in water, such as in air conditioner cooling towers. The disease is spread when mists of the water are inhaled.
Water operators are primarily interested in this pathway. The stomach and intestines are the usual targets.
The symptoms of waterbourne diseases are shared by diseases with many other transmission modes.
| Symptom | Cold | Flu |
|---|---|---|
| Fever | None or low grade | Often high |
| Chills | Rare | Common |
| Headache | Rare | Common |
| Body aches | None or slight | Often severe |
| Fatigue | Mild | Can be extreme |
| Cough | Mild/moderate | Dry, hacking |
| Runny/stuffy nose | Common | Sometimes |
| Sore throat | Common | Sometimes |
| Sneezing | Common | Rare |
Your customers becoming sick may be an indicator of a problem with the water supply. This is a poor indicator, since major damage will have been done before you receive notification. Doctors are required to report to the Health Department a wide range of communicable diseases, including giardiasis. The health department will attempt to isolate a common cause (perhaps a food, restaurant, pool, or the water) so that the problem can be addressed.
Also known as heterotrophic plate count. The test counts bacteria of many types, but does not identify any particular species. Usually expressed in colonies per milliliter.
Three different tests are available for coliform bacteria. All of them grow the bacteria on special media (nutrients). Results are usually in colonies per 100 milliliters.
The Most Probable Number test (MPN) uses several test tubes with differing dilutions of sample water. It yields an estimate of the number of bacteria in the sample (the most probable number of bacteria). MPN is more difficult for laboratories to perform, but is less susceptible to interference from turbidity.
The Membrane Filter Test (MFT) catches the bacteria in the sample on a filter (the pores of which are smaller than a bacterium but larger than water). The bacteria are then counted. Naturally, turbid water will clog the filter, making the test unusable.
The Presence/Absence test (P/A) uses one tube and just tells if coliform bacteria are present. It does not tell how many were found. This is the easiest test.
In most cases, your lab should automatically check for E. coli if any coliform is found.
Trihalomethanes (THMs) are formed when organic matter in the water reacts with chlorine disinfectants. THMs can cause cancer.
Lead is the worst of many metals that cause health problems. Mercury, cadmium, and arsenic also have long histories as poisons.
From septic systems (the breakdown of ammonia in sewage) or fertilizers. Nitrate interferes with breathing of infants and small children.
When the contaminant is known, direct testing is available and practical. Because there is a limited number of metals direct testing can be practical. Like the biological agents, organic chemicals are innumerable. Certain classes can be tested for, but indirect or indicator type tests may be necessary.
These series test for a number of similar chemicals. Often, organic contamination is composed of a mixture of specific chemicals or the contaminant breaks down into several different chemicals. Maybe one or more of them will be detected by the series. The series for testing drinking water are numbered in the 500's. Do not use 600's (surface or sewage) or 8000's (soil or solid waste) numbered EPA series. These series do not correspond to the groups in the sanitary code.
The human body has very sensitive chemical analyzers (the nose and mouth). Humans are better at detecting types of molecules rather than identifying specific ones. Human sensitivity varies from person to person and from hour to hour.
Disinfection is the process by which water and water systems are made free from pathogens. Sterilization is the destruction of all bacteria, virus, etc (all living things).
Chlorine can act as an oxidizer, one of the three necessary components for fire. If fuel and spark are also present with chlorine, fire will result, even without oxygen. Starving such a fire of oxygen will not help.
Standard kits are available for common problems with standard size tanks. Always be familiar with use of safety equipment prior to needing it. You will not have time to read the directions while your tank is leaking.
Reactions favor some distribution of chemicals. Some reactions favor one side strongly, others are more even.
If some stress is brought to bear on a system in equilibrium, a change occurs, such that the equilibrium is displaced in a direction which tends to undo the effect of the stress. In other words, a reaction will compensate increases in concentration of constituents on one side of the equation by forming more of the constituents on the other side.
The speed at which a reaction proceeds. This is independent on the equilibrium point (or constant) of the equation. A reaction which is very favorable may or may not proceed quickly. The speed of reactions is important in designing treatment processes. The reaction of chlorine and hydrogen sulfide is quick, so a detention tank may not be necessary. The reaction of chlorine and bacteria is slower (and dependent on temperature, pH, and the type of bacteria) so a detention tank is necessary to provide time for the bacteria to be killed prior to entering the distribution system.
Water is composed of two hydrogen atoms bonded to one oxygen atom. When water splits up (dissociates) two ions are produced, the positively charged hydrogen ion and the negatively charged hydroxyl ion. The hydrogen ion is what pH measures. In pure water enough of the water dissociates to produce hydrogen ions in an amount which measures 7 on the pH scale. Although pH is a measure of the concentration of hydrogen ions, its units are not typical concentration units, like mg/l. A mathematical formula is applied to the concentration to yield a scale which ranges from 0 to 14. On this scale 7 is neutral; lower numbers represent acids; higher numbers represent bases. Bases are also called alkalis or caustics.
Acids are chemicals which produce hydrogen ions (H+). A solution which is acid has more hydrogen ions than a basic solution. Remember that the pH will be lower.
Water dissociates to the hydrogen ion and the hydroxyl ion. The equilibrium point of this reaction is to have 10 quadrillion times more water than ions. Notice that equal numbers of H+ and OH- ions are produced in this reaction. The H+ ion, or hydrogen ion (also called a proton) is the chemical measured by pH.
Hydrochloric acid dissociates into the hydrogen ion and a chloride ion. This reaction strongly favors the right side. This is why hydrochloric acid is a strong acid.
Hypochlorous acid dissociates into a hydrogen ion and hypochlorite ion. The balance point of this equation is determined by the pH (the concentration of hydrogen ions.)
Chlorine gas mixed with water forms hydrochloric acid and hypochlorous acid. The hydrochloric then dissociates (see above) lowering the pH. Hypochlorous acid (and the hypochlorite ion) are known as free chlorine, free residual or free chlorine residual.
Sodium hypochlorite added to water forms the sodium ion and the hypochlorite ion.
The hypochlorite ion from the previous equation can take a proton (hydrogen ion) from water to form hypochlorous acid and the hydroxyl ion. In an acid environment the hypochlorite ion consumes a free hydrogen ion. This raises pH since hydroxyls are created or hydrogen ions are used up.
Chlorine works much better at low pHs.
More trihalomethanes are formed at high pHs.
Chlorine is more effective at higher (warmer) temperatures, however, it also breaks down faster so should be stored at low temperatures. Light also hastens the destruction of chlorine.
Chlorine can combine with ammonia or organic nitrogen to form chloramines. This is known as combined chlorine or combined residual.
Chlormamines are more stable than free chlorine.
Combined chlorine retains some disinfection potential but is not as effective as hypochlorous acid. Organic chloramines do not have any disinfection potential.
Chlorine reacts with bacteria (to kill them) and with many other chemicals or contaminants in the water. You must use enough chlorine so that some is left over (the residual) even after the chlorine has reacted with impurities. Because chloramines are less effective disinfection agents always measure free chlorine residual.
Chlorine also breaks down of its own accord (see temperature dependence, above). The time that the water takes to reach the end of the distribution system will thus exert a sort of chlorine demand. Long distribution systems or poor quality water may require intermediate chlorinators to be installed in the distribution system
Breakpoint chlorination is used to remove ammonia from the water. Most water sources should not have ammonia present. This technique is primarily applicable to sewage treatment.
Breakpoint molar ratio is 1.5 hypochlorous acid to 1 ammonia. Ratio is 7.6 mg hypochlorous acid to 1 mg ammonia. From the equation above, we can see that the process is dependent on the pH of the water.
If you dilute a volume of solution, the product of the concentration and volume of the undiluted solution will equal the product of the concentration and volume of the diluted solution.
Be sure to clearly designate the two solutions you are equating. One might be in the crock, the other in the commercial strength bottle. One might be in a tank. One might be in a pipe. Use subscripts to help you: CbottleVbottle = CtankVtank.
One way to look at dilution is to think separately about the chlorine and the water. A volume of liquid with a concentration of chlorine has a fixed amount of chlorine equal to the volume times the concentration: CV=mass. Adding more water will not change the mass of chlorine. Thus we can use the known mass and the new volume to figure out the new concentration:
mass / Vnew = Cnew.
Rearranging C1V1=C2V2 to V2/V1=C1/C2 reveals the dilution factor (C1/C2). To obtain a solution one tenth the strength of the original solution you would dilute by a factor of ten. For example, to obtain 1% solution from 15% solution you would dilute by a factor of 15 or one gallon of 15% in each 15 gallons of total solution (to fill a 50 gallon crock you would use 3 gallons of 15% solution and 42 gallons of pure water.)
The feed rate calculation is just a dilution equation with time factored in. Therefore, instead of volume, we use flow (which is volume/time).
Again, keep track of each side of the equation. Instead of using the subscripts 1 and 2, use letters or words to describe each place. E.g. CcrockVcrock = CpipeVpipe.
Use the CV=mass form of the dilution equation for solid (calcium hypochlorites) or pure chlorine (either gas or liquid). Use CV=CV when the chlorine is already dissolved in water (bleach, aqueous hypochlorite solutions).
Example: To chlorinate a 20,000 gallon tank to 50 ppm requires (0.02 million gal x 50 ppm x 8.34 lbs/gal) = 8.34 lbs of chlorine.
Notice that the conversion factor 8.34 lbs per gallon is used to convert from volume to weight. Notice that the parts per milllion cancels with the million in the million gallons. Remember that ppm and mg/l are equal (for dilute solutions).
Same problem, conversion factors split up: 20,000 gal x 50 mg/l x g/1000mg x kg/1000g x 2.2kg/lbs x 3.78 litres/gal = 8.34 lbs of chlorine.
lbs=mg/l*8.34*MGD
For calcium hypochlorite remember that only 70% of the weight is from hypochlorite, the other 30% is calcium. In the above example 1.42 lbs of calcium hypochlorite would be necessary (since 70% of 1.42 lbs is 1 pound).
Feed to the center of the pipe. Water flow in the center of the pipe is faster and more turbulent. Water flow at the wall of the pipe is slower and less turbulent. Use several (10) pipe diameters for turbulent mixing. Inline mixers can be used to ensure proper mixing when space is limited.

Dilution equation
CV=CV
chemical mixing
estimating service time
T = V/Q
These pumps are designed to pump the same quantity regardless of the pressure against which they pump. Since the flow rate is known, the quantity pumped can be determined. Positive displacement pumps are also called metering pumps or chemical feed pumps.
Antisiphon valve
The chlorinator insures that hypochlorite solution is pumped into the water when the the chlorinator is on. The antisiphon valve insures that no hypochlorite solution is sucked into the water when the chlorinator is off.
Why is the antisiphon valve important?
The antisiphon valve may sometimes be used to help prime the chlorinator, to flush the chlorinator, or as a pressure relief valve. These more complicated antisiphon valves are called four function valves.
Always make sure that the chlorinator is actually primed and is pumping chlorine solution.
Always have spare parts or a spare chlorinator available.

Acid wash
Some have found acetic acid (vinegar) effective. Hydrochloric acid (muriatic) is stronger and more dangerous to use.
Lubrication
Be sure to use only food grade oils where there is the possibility that oil will enter the water system.
Bacteria require food (a carbon source) and energy (light, oxygen, other chemical sources) to grow and reproduce. Consider using GAC or other treatment to remove total organic carbon from your source water.
Ultraviolet radiation is used to "sunburn" the bacteria to death. The strength of the radiation and the length of time of exposure are factors influencing the effectiveness of the treatment. Any material, dissolved or suspended, that blocks ultraviolet light will interfere with the treatment. Ultraviolet works best on clean, clear water.
Quartz blocks less ultraviolet energy than ordinary glass.
Ozone or O3 is a colorless gas formed from three oxygen atoms. The molecule is unstable and a powerful oxidizer.
The same characteristics that make ozone (and all disinfectants) toxic to microorganisms also make ozone toxic to humans. Ozone Exposurei
| Detectable odor | 0.01 to 0.05 ppm by volume |
| Coughing & irritation in 8 min | 1.0 |
| Coughing & irritation in 1 min | 4.0 |
| Fatal in < 1 min | 10,000 |
| OSHA 8 hour limit | 0.10 |
| OSHA 15 minute limit | 0.30 |
O3 + contaminant <--> O2 + oxidized material
Ozone does not produce a residual. A chlorination system is normally used to provide a distribution system residual.
Aldehydes, bromate Partially oxidized organic matter (lower molecular weight compounds, more polar compounds, aldehydes, ketoacids, ketones) which are more biodegradeable. More food means more problems with regrowth in the distribution system. Ozone should be followed by filtration.
| Treatment | |||||||||||||||
| Contaminant |
Ion Exchange |
Air Stripping |
Chlorine |
RO (membrane) |
Filtration |
Chemical addition |
UV |
||||||||
|
Common names |
Cation |
Anion |
|||||||||||||
|
Radium |
10 percent Anion resin added. Regenerate with KCl preferred. |
v. good |
|||||||||||||
|
Radon |
BAT |
good (can't dispose?) |
maybe |
||||||||||||
|
Fe |
rust |
fair |
V.Good |
follow with settling/filtration |
bad |
should pre treat |
with pre oxidation |
polyphosphates and/or oxidants |
|||||||
|
Mn |
black stains/flecks |
BAT |
too slow |
bad |
should pre treat |
with pre oxidation |
polyphosphates and/or oxidants |
||||||||
|
Other metals |
maybe |
yes |
|||||||||||||
|
Pathogens |
bacteria, viruses |
BAT, turbidity interferes |
maybe not viruses |
for protozoans and cysts |
chlorine |
fair |
|||||||||
|
Hardness |
Calcium, Magnesium |
BAT |
good |
polyphosphates |
|||||||||||
|
Nitrate |
fertilizer, sewage |
fair |
good |
||||||||||||
|
Chloride |
salt |
only choice |
|||||||||||||
|
Color |
KMnO4 may oxidize colors |
may oxidize color |
good |
maybe |
oxidants |
||||||||||
|
Odor |
maybe |
KMnO4 may oxidize odors |
may oxidize odor |
maybe |
maybe |
oxidants |
|||||||||
|
Organic |
gasoline, solvents, pesticides |
smaller, lighter, more volatile POCs |
makes problem worse |
BAT |
check contaminant to membrane compatibility. |
||||||||||
|
Turbidity |
dirt |
maybe |
overkill |
good |
|||||||||||
|
Sulfide |
rotten eggs |
good |
good |
good |
poor |
oxidants |
|||||||||
|
Corrosivity |
dissolved pipes, lead & copper, asbestos |
makes worse |
alkalinity, orthophosphates, silicates, pH adjusters |
||||||||||||

Granular Activated Carbon is produced by heating wood (or other carbon containing material) in the absence of air (oxygen), much like how charcoal is made. GAC is cooked longer, so that all the hydrogen and oxygen is driven from the wood. Any other organic materials are also broken down. The cooking process causes the carbon to develop millions of tiny cracks and crevices.
See also the GAC primer for additional operation, design, and sampling information.
Contaminant particles enter the crevices and stick.
Three removal mechanisms:
Some contaminants attach more tightly than others in the crevices. Floating particles stick. Stuck particles unstick. When a contaminant is released from one crevice, it flows with the water further into the filter, where there are more open crevices to get caught in. This is the most important removal mechanism and the main reason why GAC is used.
GAC filters also function as depth filters because the carbon comes in 'granules'. Although the granules are relatively large, however, a prefilter is still recommended.

Unlike most filter media, GAC is not inert. The carbon can be oxidized. Strong oxidants like hypochlorous acid are completely removed by activated carbon. Therefor, you cannot disinfect a GAC filter. You must have post-chlorination.
Chemical tests are the only way to see if breakthrough has occured.
Use pressure gages to check for plugging.
Contaminants of the proper size to fit into the cracks in the carbon will not cause head loss. (The granular bed will also act as depth filter so larger particles may be trapped in between the granules and increase the filter head.) The best way to gauge when activated carbon needs to be changed is to find "break through", that is, contaminant in the filter effluent.
In order to protect the customers, a minimum of two activated carbon filters in series are always used. When break through is detected after the first filter, the second filter is put in the first position and a brand new (virgin) filter is put in the second position.
If filters are used in parallel, it is important to check that each unit has the same head loss, otherwise, one "train" will treat more water. Use flow control valves (Griswolds) to ensure even flow between parallel filters.
Never backwash activated carbon. It is unlikely that sufficient clean water is available to remove even a small portion of the contaminant lodged in the crevices. Additionally, mixing up the carbon (think bed expansion) will put highly contaminated granules from the "top" of the filter near the "bottom" (is your filter upflow or downflow?) where they will release contaminant into the finished water.
Because activated carbon can not be backwashed, it is advisable to put a prefilter before the carbon to prevent particulates from plugging the activated carbon (thus creating a very expensive depth filter).
The carbon or the filter vessel with the carbon are usually trucked off site to a disposal or recycling facility.
Contaminants which dissolve in air, such as gasses or volatile organics, can be removed by allowing them the opportunity to transfer from the water to air. Large quantities of air and/or small bubbles provide a large transfer surface.
Air is bubbled through the water to remove dissolved gasses (other than air).
Since the spent air is vented to the atmosphere, this process reduces the pressure of the water to atmospheric. Booster pumps are necessary after the air stripper.
Henry's Law
The air to water ratio can be adjusted.
Discharge of contaminant to the air may be regulated.
Ions are attached to a substrate (Zeolite or synthetic beads). As water is passed over the beads, the attached ions are displaced by alternate ions in the water.
Make sure that this is away from any water sources. Chloride is not attracted to soil particles so will not be removed.
Reverse osmosis is effective in removing most contaminants, including ions, organics, and bacteria. It is, however, expensive and complicated. RO is not effective in removing small uncharged molecules. Certain contaminants may deteriorate the RO membrane. Removal efficiency varies.
Membranes are optimized for specific contaminants and water conditions. Not all contaminants are removed by all membranes.
Osmosis, based on diffusion, is the tendency for water on the clean side of a semi permeable membrane to migrate across the membrane to the contaminated side. By pressurizing the contaminated side, the process is "reversed". In practice contaminated water flows along one side of the membrane. Some of the water filters through the membrane thus increasing the concentration of contaminant on the influent side, the concentrated flow, called reject or brine, must be disposed of. So, reverse osmosis splits influent water into a pure stream and a concentrated stream.
Most important for low pressure applications
If a membrane is being used to remove biological agents then holes or tears in the membrane or gaskets will destroy the effectiveness of the membrane.
Holes or tears will also degrade removal efficiency of other compounds.
Direct monitoring
Air pressure hold testing
sonic testing
Bubble point testing
Indirect testing
Particle counting
turbidity
The influent pressure, the flow rate, and the ratio of clean water produced to reject water produced can be varied in order to obtain different effluent qualities or removal percentages.
Greensand is used to remove oxidizable ions, particularly iron and manganese.
Green sand filters use permanganate to oxidize the contaminants to insoluble (filterable) forms or to inert forms. The permanganate is bound to the filter media (special sand). The permanganate gives the media a greenish appearance.
For batch systems, consider ORP?
For continuous feed systems, the pinkness in the output water indicates over feeding of potassium permanganate. ORP can be used and can control the permanganate feed.
The feed rate of KMnO4 can be adjusted.
See above.
Zinc or phosphates can be used to coat pipes to inhibit the water's dissolving of the pipe material.
Phosphates, caustic soda, bicarbonates may be used to adjust pH or alkalinity to reduce the corrosivity of the water.
Various fluoride containing chemicals are injected into the water to raise the fluoride level. See below.
See Green Sand or Ion Exchange.
Any oxidizer may convert dissolved Fe or Mn to insoluble forms. Then filter. Weaker oxidezers may require contact times which are too long to be practical.
A chemical is mixed with the water. Provision must be made to ensure adequate mixing and contact time so that the chemical will be effective.
Usually, the chemical reacts with contaminants in the water to inactivate, destroy, or otherwise prevent the contaminants from doing harm.
Some chemicals are intended to form a coating on the plumbing system.
Set metering pump rate (stroke and frequency). Metering pump may be controlled automatically based upon feedback from sensors.
Order and relative location of injection points is very important when more than one chemical is added.
Chemical feeders are normally set to inject chemical into the raw water line from the well. The feeder is put on the same electrical circuit as the well pump. Since the well usually delivers a constant flow, a fixed chemical dose can be added.
Warning: if the well pump fails mechanically or in any other manner where the control panel does "know", the chemical feeder will be pumping but the well will not. A very high dose of chemical will be delivered when the well pump is repaired (or, perhaps, reset). Consider flow switch interlocks on chemical feed pumps.
Test kits or meters can determine residual of injected chemical. Be careful that injection pumps do not lose their prime.
Adding chemicals to the water generally doesn't create waste at the water treatment facility, but may cause problems for the end users of the water. A common problem arises when zinc compounds are used for corrosion control. The extra zinc in the water can raise the level of zinc in the sludge produced by downstream sewer treatment plants, increasing sludge disposal costs.
There are few adverse health effects from iron or manganese. Very high levels of manganese may cause health problems. The amount of iron present in water is too little to provide nutritional value.
Iron stains fixtures and laundry reddish brown. Manganese produces black stains. Bleach "fixes" these stains. Fabric life is reduced.
Rust or manganese precipitate deposits in boilers, mains, valves, and other tanks. The deposits can prevent proper valve closure, reduce pipe and tank volume, and otherwise prevent proper operation of equipment.
Iron and manganese exert a chlorine demand, i.e. they will be oxidized by chlorine. This uses up chlorine which might otherwise be disinfecting. Iron can be an energy source for bacteria. The so called "iron bacteria" are colorful, produce tastes, odors, sheens, and slimes, and are very difficult to kill.
Fe2+ and Mn2+ are soluble. Iron has two oxidation states, +2 and +3. Manganese has many.
In general, the higher oxidation states are less soluble.
Are there stains? Is the water brown? Are there black flecks? How does the water look when you flush a hydrant?
Add a few drops of chlorine to oxidize dissolved Fe/Mn. Watch for rust or black flecks of Manganese to form and settle to bottom. Look for brown color.
Be careful when sampling to use properly preserved bottles to prevent Fe/Mn from precipitating out on walls of container. Be careful when sampling to note particles. Particles of iron or manganese that get into the sample will greatly increase the result. Clays also contain substantial amounts of Fe/Mn. As with all sampling, your Fe/Mn sample should be representative of the water you wish to test. Do you want to be testing the particulate matter? You can filter the water prior to testing to determine whether the iron/manganese is soluble or insoluble
Need Spectrophotometer, filter, or Nessler tubes to compare color of reacted sample.
Large expensive delicate laboratory machine.
Produces most accurate results.
Survey other wells in area. Upper aquifers may be better if they are aerated enough to precipitate out iron
Block off iron/manganese rich vein of water. Call your well driller.
Avoid this method.
Prevents oxidation (and precipitation) of Fe/Mn. Does not remove Fe/Mn. Requires addition of chemicals (Pyrophosphate, metaphosphate, tripolyphosphate). These can be nutrients and promote bacterial growth. Check phosphate limits at the local sewer plant. Break down to orthophosphate. Bench test to approximate amount. Observe color. Should stay clear for 4 days.
Add phosphate before or with chlorine. Best right below well pump.
Must keep oxygen out of resin (or Fe/Mn will plate out on resin beads. Use sulfites during backwash. Pump to waste when starting feed pumps.
removes about 60-80%
requires little attention
record filter volumes
Slow unless pH > 6.5
Effective for iron. Not effective for manganese. Use Ozone, Chlorine Dioxide, or Permanganate.
Remember that Greensand is both an oxidation and a filtering process. Other oxide coated filter media may be substituted for real Greensand.
See Disinfection, above.
Turbidity is caused by materials in suspension that interfere with the passage of light. Turbid water appears cloudy. Turbidity itself does not necessary cause disease, but can create real problems
Rather than drink turbid water, consumers may choose untested or unsafe supplies. Your reputation is tarnished
Particulates shield microorganisms from disinfection
Particulates clog filters
Clogs the membrane used in certain coliform tests.
Changes apparent color in chlorine and other color change tests.
See surface water treatment, below.
Put air stripper in front of GAC when treating heavily contaminated waters.
GAC is marginally effective on MTBE. Total removal is possible, but filter runs are very short. In other words, time to breakthrough is short.
Best Available Treatment for MTBE. Most small systems use GAC anyway since initial cost of GAC is lower.
Anion exchangers replace nitrate with chloride. Be careful that your chloride limits are not exceeded since other anions (carbonate) will also be exchanged.
Triethyl-amine and tributyl-amine are nitrate selective.
Hardness does not directly cause health problems. Hardness reduces soap lathering. Hardness deposits calcium or lime equipment in contact with the water. These deposits are difficult to clean, clog pipes, jam valves, and interfere with or damage water heaters.
If the water is too soft, it may dissolve plumbing, causing leaks and/or contamination in the finished water.
Ideal harness is 70-100 mg/l as CaCO3.
Blend water after ion exchange type softeners, since these remove all hardness.
Special softening membranes don't remove viruses.
Iron/Manganese? Sand/silt/clay?
Cloudiness clears gradually, starting at bottom.
Dissolved air is coming out of solution due to temperature or pressure changes when water leaves faucet.
More likely during cold weather because gasses are more soluble in cold water.
Not a health concern. Some appliances may not function properly if air becomes trapped in their plumbing.
Normally settles to the bottom of a glass.
Aluminum oxide from the reaction of some waters with the sacrificial aluminum anode in some (older) water heaters. Should be present in the hot water only.
Replace water heater or anode.
Very fine silts and clays can be suspended in water giving the water a cloudy appearance.
Consider well redevelopment, redrilling, or recasing. As a last resort, consider filtration.
2-methylisoborneol (MIB) and trans-1,10-dimethyl-trans-9-decalol (geosmin) produced by blue-green algae and actinomycetes are detectable by nose at 9 and 4 ppb in water.
Check ecology of surface water source.
MIB can be removed by PAC. Chlorinate afterward. Chlorine oxidizes absorption sites and causes already absorbed MIB to be released. Ozonation followed by biofiltration may be more effective than PAC, chlorine or potassium permanganate. Ozonation alone partially removes MIB and geosmin but causes other problems.
Check residuals, including combined. Check dosing. Check raw water for iron, manganese, ammonia and other chlorine demanding chemicals.
Kerosene, or possibly MTBE (MTBE can cause odor at concentrations as low as 5 ppb). Might be caused by chlorine dioxide disinfectant reactions. Lower dosage/increase purity of chlorine dioxide.
Might be caused by chlorine dioxide disinfectant reactions with new carpet. Lower dosage/increase purity of chlorine dioxide.
Check iron and manganese
Shiny black flecks are oxidized manganese.
Check well pumping level, well screen, gravel pack, well yield and pump rate.
High pH (alkaline) water can feel slimy. Check pH. Check chemical feeders.
Soaps do not lather well in hard water. Soaps are hard to remove with soft water.
See chlorination.
Solve epidemiologically.
Water is known as the universal solvent.
Fluoride in the water makes teeth harder, which prevents cavities. A significant savings in dental bills results. Most areas have a low natural fluoride level (0 - 0.1 mg/l).
Too much fluoride in the water can cause blackening of the teeth and tooth loss. Problems usually occur at fluoride concentrations above 4.0 mg/l.
The US EPA has promulgated and the NYS Health Department, Bureau of Public Water Supply Protection has included into Part 5 of the State Sanitary Code, requirements for filtering of all surface water supplies, unless those systems are able to meet certain avoidance criteria. This rule is known as the Surface Water Treatment Rule (SWTR). Due to the financial burden this rule would impose on small water systems if conventional filtering methods were installed alternative filtering methods have been developed. These field tested alternative methods are available to small water systems for use in complying with the SWTR. Part 5 of the State Sanitary Code states that any addition or deletion of a treatment process must be approved by the State. Therefore, those Grade C systems which must filter will need treatment system plans prepared by an engineer, and submitted to the health department for approval. This must be done before any changes can be made.
Should any bacteria or cysts remain after filtration, a disinfection system will act as a second barrier to distribution system contamination. Filters can be excellent places for bacteria to colonize, therefore, disinfection is required after all filtration systems.
The purpose of filtering surface water is to provide a potable water free from turbidity, color, and which is biologically safe to drink. Surface waters sometimes contain a parasite known as Giardia Lamblia. The Giardia Lamblia cysts enter the water from the feces of animals such as beaver, deer, dogs, and even humans who may be infected with the parasite. Drinking water that contains the cyst can transmit a waterborne disease to consumers. When the cyst enters the body, the disease that develops is know as giardiasis. Giardiasis is not usually life threatening, but can be incapacitating. These parasites attach themselves to the wall of the small intestine, grow and reproduce there. Disease symptoms include diarrhea, flatulence, abdominal cramps and loss of appetite.
Items that influence raw water quality:
Large water systems using surface waters normally can remove the Giardia cysts with a series of treatment steps called conventional filtration. In conventional filtration the water and a coagulant, such as aluminum sulfate, are mixed together in a rapid mix chamber. The water is then slowed down for flocculation, where the coagulant and "dirt" particles combine to form larger particles called floc. After flocculation, the water's velocity is decreased further in a clarifier or settling basin where the floc settles out. The water then goes through large filtering systems such as rapid or slow sand, diatomaceous earth (DE), or multimedia filters to remove remaining particulate matter, including the giardia cyst. Disinfection is then performed using chlorine, ozone or U-V to kill any micro-organisms that may have penetrated through the filters. Upon clogging, the filters used in conventional filtration require complicated backwashing cycles to regenerate their capacity.
Conventional filtration is complicated, so requires expert knowledge to operate. The size and number of processes also make it very expensive. Cartridge or bag type filters are a simpler and affordable alternative for smaller systems. In cartridge filtration, the water is applied directly to the filter. No chemical addition, coagulation, flocculation or settling is performed. The filter element is self contained and is replaced rather than backwashed.
Strainers
Some cartridge filters work on the principle of straining. The filter has tiny holes. Particles bigger than the holes can't pass through. Smaller particles fit through the holes and are not trapped. The particles passing through the filter show up as turbidity in the filtered water.
All bag filters are of this type.
Depth
Some filters work on the principle of adsorption, or sticking. The filter is filled with a media in which particles can become trapped. These medias include sands and wools. They are sometimes called depth filters, perhaps because the contaminant accumulates into the media rather than at the surface, as in a strainer type filter.
Activated carbon
Activated carbon traps molecules in small crevices in the carbon. Carbon is useful for removing tastes and odors. Carbon comes as a powder or as granules, so will also function as a depth filter (albeit an expensive one). Do not confuse activated carbon with other carbons (coals or anthracites) used as media for depth filters. Only activated carbon can remove the extremely small agents causing taste and odor.
Filters are rated by the nominal size of the particles they can remove. Straining type filters usually remove most particles bigger than their pore size. Depth type filters remove some particles which are smaller than their rated size and also allow some bigger particles to pass through.
The removal efficiency is related to the size of the filter opening, usually listed in microns, and the type of filter media.
Any filter you purchase should be rated for efficiency. Efficiency is the percentage of particles of the rated size that the filter removes. Some filters are tested for efficiency by using a silica test dust in water at 4 gpm; others by using Arizona sand in water. In both tests, efficiencies are calculated using particle counts, not mass weight. The test is conducted using a fresh filter that has been operated for 10 minutes. The efficiencies are specific to the particle size tested, and do not include those of other sizes. If a filter is not rated for efficiency it should either be used only as a pre-filter, or not purchased at all.
Since all cartridge and bag filters are rated in MICRONS, let's take a look at what microns are; The dictionary defines micron as a "unit of length one/one millionth of a meter." A better example would be the period at the end of this sentence is approximately 400 microns. Now reduce this to a 1, 5, 10 micron size, which is normal for these types of filters. Micron size is also related to flow rate, the smaller the micron size, the slower the flow rate through the filter. The smallest pore size available is one micron. Smaller sizes are usually regarded as membrane filtration.
A cartridge filter consists of a filter module which fits into a (usually) cylindrical housing.
Filter
Cartridge filters are usually made from the following materials: Cellulose, cellulose/polyester, polypropylene, cotton fiber, granular activated carbon, powered activated carbon, phosphate crystal and ceramic. The micron rating will vary from 100 down to 1 micron. Granular activated carbon filters do not have a micron rating. The powdered activated carbon may have a nominal rating of 1-5 microns.
Housing
Filter housings are made of stainless steel, or high density plastic. They are manufactured in single or multiple cartridge models. On those models that have a bottom discharge, an automatic air relief valve is required to vent air that may become trapped in the filter housing. This air may accumulate from degassing of the raw water.
Multiple units (trains)
Often several cartridge filters are arranged in a series of filters set in descending order of filtration sizes, e.g., 10 micron first, 5 micron and last a 1 micron. The first filter traps the largest particles; the next filter has only to filter the remaining smaller particles. This allows each filter to remove the particles for which it is best suited, resulting in longer filter runs.
All filters should have good end seals. This is especially critical with the final filter. If the end seals do not seat tightly against each end of the filter canister, "short circuiting" will occur. This phenomenon occurs when the water flows over the top and bottom of the filter, and not through it.
When choosing a system for filtration, it is necessary to select a system that offers cost effective benefits. The following economies can be gained by selecting the most effective and efficient system.
| Long Runs | Low labor |
| Disposable | Quick changes |
| Reusable | minimize purchases |
| Low pressure drop | Smaller pumps, lower power costs |
| Small size | cheaper building |
| Approved | or else |
Intake/Pretreatment
Some pretreatment is going to be required just prior to the filtration process. Poor pretreatment can result in extremely short filter runs and frequent filter replacement. This pretreatment can be one of four types:
1. An infiltration gallery which would be located underwater approximately 20 feet off shore;
2. A well point driven into the lake bottom, approximately 20 feet off shore;
3. A shallow on-shore well, where possible and practical, could be used;
4. A "simple" slow sand filter, measuring approximately 4 X 8 feet, could be built using a good exterior or marine grade plywood. The filter when complete would have an under drain system, a layer of graded gravel and topped with a layer of sand. Topping this off would be a layer of solids and biological growth called schmutzdecke which forms on top of slow sand filter after it has been in use for a while.
Prime mover
Since filters require medium to high pressures to operate, gravity is generally insufficient to move water through the treatment plant. Pumps must be used. It is critical to the successful treatment scheme, that all the pumps be properly sized. Improperly sized pumps cause some or all of the following: energy waste, extra pump wear, inability to maintain system flow or pressure, or filter damage.
Disinfection
Should any bacteria or cysts remain after filtration, a disinfection system will act as a second barrier to distribution system contamination.
Filters can be excellent places for bacteria to colonize, therefore, disinfection is required after all filtration systems.
Replacement
A pressure drop or an increase in turbidity in the finished water will tell the operator that the filter needs replacing. Make sure that your water system has appropriate gauges and that they are functioning. The water operator is cautioned to pay particular attention to their raw water turbidity meter readings, since a turbidity spike could blanket out and clog a filter. Also, since the source is a surface source, the cartridge filter performance will be influenced by algae. Algae are microscopic plants, observed as "green scum" growing on rocks and docks, etc. Algae can clog the filter system, thus preventing a ready supply of raw water from entering the filters. Algae may suddenly "bloom" when weather conditions are favorable. A properly operating and maintained pretreatment system will reduce loading on the cartridge filters from high turbidity or algae in the raw water.
Beware of changing cartridge brands or types. The cartridge must exactly fit the housing in order to prevent short circuiting or bypassing.
A good ceramic cartridge filter will cost in the neighborhood of $40. The danger here is that if the filter ever has to be replaced, it is usually replaced with a cheaper filter. The cheaper filter may result in "short circuiting," because it will be less efficient and require more frequent replacement.
Reuse
Of all the filters named above, the only one which is reusable is the ceramic filter. This filter when clogged may be cleaned with a stiff nylon bristle brush or by blowing the dirt from the openings using an air compressor. Cleaning with air will probably do a better job.
Spare parts
Spare filter cartridges should always be available.
Disinfection
When using cartridge filters or bags, the chlorination system MUST be continuously adjusted. This is because the flow through the filters decreases as the filter become clogged. The best type of chlorinator would be the "flow sensing" chlorinator. Flow sensing (or flow paced) chlorinators automatically adjust the chlorination rate based on information from the system flow meter.
Reporting
Surface water supplies are required to fill out and submit additional forms beyond the standard Operation Report. Please note that water supplies using filtration are required to meet strict finished water turbidity requirements. See Part 5.
The filtration system may in the future have to filter for cryptosporidium. If so, an additional filtration system will be necessary, since cryptosporidium is too small to be removed by standard cartridge filters.
Outbreaks of cryptosporidiosis resulting have occurred at supplies using surface water when the finished water turbidity was in the .9-2.0 NTU range. The peak turbidity during the 1993 Milwaukee outbreak was 1.7 NTU. AWWA recommends that finished water turbidity be kept below .2 NTU.
Make sure that the backwash does not form a cross connection.
Used when a known quantity of liquid needs to be delivered such as when adding chemicals. Most positive displacement pumps found in water plants are piston or diaphragm pumps, but tubing (also called peristaltic), vane, and gear pumps also exist. Diaphragm and tubing pumps have no seals - water is completely isolated from the motor - a considerable advantage when pumping corrosive solutions or using toxic oils.
piston or cavity
diaphragms
vanes
gears
tubing
check valves
A cavity is enlarged, sucking fluid in. The cavity's volume is then decreased, forcing the water out.
What happens if a positive displacement pump is pumping into a closed container (or the valve on the outlet is off)?
head vs. flow
The flow is mostly independent of the output head.
impeller
casing (volute)
seals
packing
lantern rings
glands
wear rings
bearings
theory of operation
The impeller of a centrifugal pump slings the water out away from the center of the pump. The casing collects and channels this water toward the outlet. New water is sucked into the center of the pump to replace the water which was pushed outward.
The larger the impeller diameter the more pressure a pump can develop.
What happens if a centrifugal pump is pumping into a closed container (or the valve on the outlet is off)?
priming
priming water 5-1.29
head vs. flow
Based on the dimensions, motor speeds, and fluid characteristics centrifugal pumps have a characteristic pump curve which shows the head to flow relationship. If the pump is not operating on the curve, then something has changed. Perhaps the impeller is worn, damaged, or improperly installed. Perhaps the motor is not powerful enough to maintain speed.
efficiency
The design of a pump influences its efficiency. Pumps efficiency varies over the operating range of the pump. The operating point of greatest efficiency is also the point of least stress and wear.
gauges
Inlet and outlet pressure gauges let tell you the suction head and pressure head on the pump.
Flow meters tell you the flow from the pump.
The above information can be checked against the pump curve to diagnose a variety of pump problems.
valves
Allow you to adjust the outlet head and thus the flow.
Check valves help to keep prime.
Submersible pumps use oil-cooled motors. The oil must be food-grade. Certain old pumps may have PCBs in their oil.
The water flowing past the motor takes the heat away from the oil. The water entering the well should be entering below the pump level so that the water will be flowing past the motor (submersible pump motors are mounted beneath the pump because it is easier to route the power wires past the pump than the pump output past the motor).
Submersible pumps that have little clearance between the motor and the well casing may not get adequate motor cooling.
The purpose of storage is to meet peak flow requirements and provide a buffer when sources are unavailable. Storage can also provide chlorine contact time and a convenient location to add external water to the system (e.g. when you truck water in).
Pressure tanks store the pressure that the pumps create.
Pressure tanks are used to protect pumps. The system use is quite variable, but pumps are most efficient in one small range. The pressure tank allows the pump to operate in it's best range and allows the pump to remain on for a reasonable time. The pressure tank and pump system provide a simple way to regulate the system pressure.
Without the pressure tank, the pumps would pump against system flow. Since this can range from near zero in the night to peak flow, the pumps are all over their curve. Their output pressure (head) varies accordingly. Furthermore, during periods of low flow, the pumps quickly develop the pressure for which the cut off switch is set. The pumps then cycle on and off repeatedly.
The pressure tank also protects against water hammer caused by the cycling of the pumps.
In the bladder type pressure tank, a membrane separates the air from the water. The membrane prevents the air from dissolving in the water. Automatic replenishment of air is not necessary. Bladder tanks are limited to smaller tank sizes. The membrane material may impart some taste to the water.
Contact tanks, also known as detention tanks, hold water so chemicals can work. Which equation allows you to find contact time from volume and flow rate?
Tanks have been made from wood, fiberglass, concrete, steel (sometimes with a glass lining).
A great many coatings have been used on tanks to protect the tank from the corrosive effect of the water and to protect the water from contamination from the tank. Some of those coatings proved to be toxic. Make sure that any coating that you select is approved for use in water systems. Check with the NSF, who approves coatings.
Always follow proper safety procedures when applying coatings. Many coatings are flamable or toxic before they are cured.
These generally under register when worn.
Don't operate above rated capacity.
Like the pumps, the meters divide the flow into known volumes, then count them.
The two main types of positive displacement meter are piston and nutating disk. In the piston meter, the water moves a piston to one side, then the flow is switched to the other side of the piston. Each stroke is a known volume. The nutating disk type has a wobbling disk.
Size to flow
high head loss
can plug
These provide a yes/no type of output. Either there is flow or there isn't. The rate of flow is not determined.
The paddle type can stick or the paddle can corrode off.
A large and small flow meter piped in parallel and chosen by automatic valves.
5-35% positive displacement
0-100% compound
50-100% turbine
Water system types are differentiated by where or how the pressure is stored.
Elevated water (in a tank) provides pressure. Used mostly in large systems. Water level in tank is controlled by level sensors or an altitude valve (pressure controlled valve).
Most common. Compressed air provides system pressure. The well pump or the transfer pumps compress air in the hydropneumatic tank as they fill the tank with water.
Water level in the tank is controlled by level sensors (probes or floats) or by a pressure switch.
Air is lost to leaks or by dissolving in the water (see milky water problems). Manual or automatic air compressors may be used to provide the make up air. Automatic air compressors are usually controlled by a pressure switch and run after the hydropneumatic tank has been filled (high water level sensor has shut off filling pump).
It is possible to operate without a gravity or hydropneumatic tank by using a pump and special valve arrangement. These systems are usually used in emergencies (like replacing the pressure tank). Often, these systems have higher energy (electricity) costs, since the pump runs all the time.
Proper pipe sizes, proper valving, flushing, looping (no dead end mains).
See the code!
Install at high and low points. Use where hydrants are inappropriate. Blowoffs at the high points are used to remove trapped air from the line. At the low points, the pipe may be drained or silt, sand, and rust may be flushed out.
Always remove strainers and aerators to prevent contaminants stuck or growing on the strainer from dislodging and entering the sample bottle thus producing a sample that is not representative of the actual water conditions.
If a sample of the aquifer water is required, then all the water in the well and plumbing system must first be flushed out. Always know which water you are sampling (from the tank, the pipe, the well, or the ground) and which water you want to be sampling.
preserved
Bottles containing preservative must not be rinsed. The preservative would be rinsed out.
Some preservatives are corrosive or toxic.
Some preservatives need to be added after sampling.
volatiles
Bottles for volatile chemicals must have no air bubbles after filling. The contaminant would partition into the air bubble and be lost when the bottle was opened.
streams
When sampling streams, open bottles beneath the surface to avoid floating debris not representative of the stream water quality. Sample upstream of bridges to avoid road salt and oils. Stand downstream of the sampling location so as not to disturb the sediment or contaminate the sample with material off your boots.
representative
Sampling points must be representative of the water being used. Avoid hydrants, dead ends, and other stagnant water that is not being used. Houses on dead end mains should be tested. Avoid dirty sampling points, if possible. Sinks full of soiled dishes or outside taps next to the ground are poor sampling points. Consider the use of caps or smooth nose sampling spigots in your pump house.
avoiding extra treatment
Make sure samples are representative of the water you want to test. Don't sample treated (softened, filtered, chlorinated, etc.) water if you want to know the quality of the groundwater.
Laboratories test known samples and pure water in order to calibrate their machinery. You can confirm your sampling technique by taking duplicate samples, trip blanks and field blanks.
Laboratory equipment is very sensitive. Very small amounts of gasoline on your hands can contaminate samples. Organic chemicals can seep through gaskets, plastics, or carry through the air. Samples have even been contaminated by freon from leaky refrigerators in which the samples were stored.
Duplicate samples should both report the same results. If not, basic assumptions about the consistency of the sample water may not be true. Other problems leading to inconsistent results are poor bottle quality control or poor laboratory technique.
Trip blanks are filled by the laboratory. If contamination is found in the trip blank, there is a problem in the storage and transportation of the sample or at the laboratory (the laboratory should check their method blank). Field blanks are filled with clean water at the same time as the regular samples are taken. Contamination in the field blank suggest sampling technique error (or transportation and storage problems during the return trip.
Your appearance and action reflect upon the water supply when you are at a consumer's residence.
Unit conversions may be necessary between your laboratory report and Part 5. Watch for results reported "As N" or "As CaCO3" and make the appropriate conversions.
Lab reports may contain special codes. Have the laboratory provide explanations of these codes. Check for trip blank, method blank, or field blank codes and corrections.
holding times
detection limits
collection requirements
bottle type
preservation
chemical
Some contaminants need to be stabilized immediately after the sample is taken. Sample bottles may be prefilled with acids or other chemicals. You may need to add some acid or other chemical at the time of sampling. Observe appropriate precautions when using and mixing chemicals.
temperature
Use coolers, ice, etc. to keep samples in the required temperature range.
Know what and when to sample.
Resamples for POCs must include entire list (e.g. 502.2), not just the compound which was found in the original sample. This is because most POCs are part of mixtures with other POCs or break down into many different products. Perchloroethene (perc, PCE, dry cleaning fluid) breaks down into TCE; 1,2-cis-DCE; 1,1,1-TCA, etc.
Anything and everything.
When parts are inexpensive, the operator is tempted to skip maintenance and replace parts when they fail. This method is only practical on the very smallest of systems.
Frequent maintenance for an item may indicate a system problem. Perhaps the part is not the correct one for the application.
A part of good record keeping, you should have a map and description of where each valve is located. Update your records whenever you perform maintenance on the valve. Landmarks (houses, curbs, trees, etc.) can move.
Count the turns of the valve handle from completely open to completely closed. For a ball valve, it should be 1/4! Next time, you'll know whether the valve is closed or whether there is a rock in the valve seat.
Yes, there are left hand threaded valves. Although some valves have indicators for "open" and "closed", some don't. Expensive and embrassing mistakes occur when you don't know whether a valve is open or closed.
Each valve must be "exercised" regularly. Otherwise, the valve may not work when you need it. Make this part of your preventative maintenance program.
Exercising is a great opportunity to update your records. Are your records of valve location, turns, and direction correct?
Don't wait for the fire company. Start near the source and work outward. Avoid water hammer by opening and closing valves slowly. Flushing velocity should be 2.5 feet/second. Keep records. Be careful to maintain minimum system pressure.
Properly route flush water. Avoid scouring customers' property. Avoid creating icy roads in winter.
Notify customers in advance so that any sediment or turbidity stirred up by the flushing won't cause problems or complaints.
This section applies to larger systems (over $125,000 revenue) and includes specific requirements for the plan. Small systems are well advised to create their own emergency plans in order to minimize distractions during an emergency. The operator's memory, although excellent normally, may not be clear when stressed by a natural or man made disaster.
A simple plan should include an inventory of all equipment so that spare parts can be ordered or emergency equipment can be properly interfaced to existing equipment. Provisions for emergency power hook up and emergency tank filling should be provided. A list of public officials, suppliers, repairmen, media, and consumers to contact should be made. The plan must be updated as equipment, names and phone numbers change.
Any plumbing connection where contaminated water and potable water can mix.
Preventing contamination from entering the public water system. Does not protect the employees in the facility. Backflow prevention devices are placed at the service connection to the building or property.
Preventing contamination from entering the on premises water system. Backflow prevention devices are placed at all potential hazard points.
Backflow occurs when contaminated water is forced into the potable water system. Backsiphonage occurs when the potable water system sucks contaminated water in.
Always keep an eye open when performing meter reading, coliform sampling, complaint investigation, or other site visits.
Dangerous or hazardous cross connections are connections where a poisonous material could enter the potable water system. Examples are hospitals, where infectious diseases and/or pharmaceuticals may be in waste products; sewage treatment plants; factories using or producing toxic chemicals.
Examples are bakeries, where oils, food colorings, or other food ingredients could enter the potable water system.

Water supply involves the transfer and transformation of large quantities of substance. Great energy is needed to effect the relocation of raw water from its natural location to its new use as clean drinking water in homes. The great power of the water and the forces which move and change it can just as easily harm the water system operator. Actually, quite a bit more easily. Respect the water. Respect the materials and equipment in your water plant.
You need to make the commitment to be safe.
You need to know the what to look out for.
Occupational Safety and Health Administration. The Department of Labor also regulates safety.
Absorbants
Besides safety considerations, check the speed and capacity of your absorbant on the target chemical.
shouldn't react with chemical
Clays react with strong acids (<2.5 pH) and strong bases (>12 pH).
Celluloses should not be used with acids.
Phenolics should not be used with concentrated nitric acid, unstable cyanides or peroxides.
compatible with disposal method
Don't landfill biodegradable absorbants (newsprint, rice hulls, cord cobs, peat)(RCRA). Incinerate them.
Don't incinerate or fuel blend clay.
Efficiency
Clay absorbs less than half its weight. Polypropylene can absorb 20 times its weight. Phenolic granules absorb 15 times their weight.
Speed
Phenolics - fast. Clay - slow.
Cost
clay, cellulose - low
polypropylene - high
Interaction with water
Cellulose floats initially, becomes waterlogged and sinks.
Polypropylene come in a hydrophobic form.
other
Clay is abrasive, but offers traction. Can be driven over.
Any area not designed for human habitation.
Examples of confined spaces include manholes, pump pits, tanks, equipment closets, trenches.
Normal air is 21% oxygen. The minimum oxygen concentration for entering a confined space is 19%. 16% O2 impairs judgment and breathing. 14% causes confusion.
Too much oxygen is also dangerous. More oxygen means fires burn faster and are easier to start. Excess oxygen may interfere with normal breathing regulation in humans. Do not enter areas with more than 23.5% oxygen.
vapors
dusts
Confined spaces may house moving equipment such as cutters, stirrers, chain drives, and valve actuators. Clearance for workers may not exist. Many tanks and other confined spaces are supplied by pipes with various liquids. Other spaces may include high voltages.
Lock out all equipment and all sources of power or product.
Do not allow confined spaces to be filled with water or other fluids. Prevent high voltages from being turned on while employees are in confined spaces. Make sure that pumps, motors, actuators, and other moving equipment can not move while employees are present.
The employee in the space should keep the key to the lock out devices. Where many workers are inside, each should have his or her own lockout kit.
Hazards created during access
The work to be performed in the confined space may cause the space to become dangerous. Examples include painting and welding which, among other hazards, may cause toxic, explosive, or oxygen deficient atmospheres.
Confined spaces are often arranged such that entering and exiting, or even just moving about within them, is difficult. It may not be possible for an injured person to leave. Rescuers may not be able to remove an injured person.
This may restrict the employee's ability to request help.
Narrow or converging architectures may trap workers.
For municipalities or larger (>10 employees) companies. Check current regulations for exact details.
Special equipment is available to make confined space entry safer. Much of the following is mandated by good sense or OSHA regulations. Also check with your facility's safety plan.
These also are calibrated based on a known gas.
Catalytic
Burn gas on catalytic bead, measure increase in resistance. Poisoned by silicones. Insensitive in low oxygen environments. Non specific. Infrared
Detect hydrocarbons. Condensing atmospheres, dirt degrade response. Paper tape
Litmus like. Solid state
Metal oxide (tin oxide) material changes resistance in presence of gas. Cheap. Sensor may last 10 years. Non specific. Electrochemical Sensor lasts 1 to 2 years. Long (hours) warm up period.
Clipped to a worker, these units send an alarm if the employee is motionless for a preset time.
Many people are buried in trenches after they die. Keep it that way. Being buried in a trench is a serious threat to life. The weight of soil on the chest prevents breathing. Burial victims suffocate. Unconsciousness occurs in about 1 minute; death in 4. Victims must be dug out by hand - nearly impossible to do given the time constraint. The best course of action is prevention.
Open-trenching has the highest number of OSHA safety violations of all heavy construction industries for the period 1999-2000; the highest number of safety violations in the utility, communications and power line construction industries for this period; and the highest number of violations of all U.S. occupations for non-compliance to OSHA safety training and education requirements.ii
Different soils have different strengths. Sands and gravels are usually strong but loose. Clays are weaker. Soils have different properties depending on their current moisture content. Wetter soils are usually weaker.
Trenches over 5 feet deep require shoring.
Stepping or sloping the trench sides will reduce the potential for collapse.
The strength of soil depends on the moisture content of the soil. Some types of soil are more sensitive than others. A soil which is strong and hard when dry may be mud when wet. Alternatively, some soils may lose strength as they dry.
Although you may be working safely in a trench this morning, changes in the soil moisture can make the same trench deadly in the afternoon.
Often, there will be no warning.
Although fire and water are opposites (in medieval chemistry, at least), either you are safe, or you aren't.
Three components are necessary to start a fire. Here are some examples which are commonly found at water treatment plants.
Building materials such as wood and steel
Filter materials such as anthracite and activated carbon
Lubricants and fuels such as oil, grease, gasoline, kerosene, and diesel
The oxygen in the air.
Oxygen trapped in activated carbon
Chlorine
Potassium permanganate (KMnO4)
Sparks
Heat
In general, this should be left to experts (the local fire company), however, you should already have met with the local fire company to explain the special hazards present at your water facility.
Use the right tool for the job! Don't hurry.
Read the manual.
Use guards. Especially shafts, shaft ends, belts, belt drives, gears, gear trains, chains and sprockets. Guards also protect machinery from errant tools and objects.
Label everything. There should be no mystery liquids or mystery valves at your plant. Don't leave your safety to chance.
The Public Service Commission (Department of Public Service) regulates the rates of private companies which sell water.
The players: E. coli O157:H7, Campylobacter jejuni, the operators, the public health officials, the customers.
The field: The wells were located in a pasture.
The scenario: Heavy rains soaked the manure spread on the field surrounding the Well 5. Well 6 was hit by lightning. Well 7's chlorination system was not working. No chlorine residuals were being taken in the system. Routine sampling of the water system was not being properly done. People became ill. When queried, the operators said there was no problem at the water system. The operators then disinfected the wells and flushed the system. The operators withheld sampling information. More people became ill, some died. A boil water order was issued by the public health authorities. E. Coli was found at the ends of the water system. After repeated denials, the operators eventually revealed that they new about problems at the water system.
The mistakes.
Failure to maintain disinfection
Failure to maintain well head protection
Failure to take representative samples
Failure to properly label samples
Failure to report contaminated samples
Failure to take appropriate action when problems were discovered
Failure to flush dead end lines.
The Lesson?
Coliform Forming Unit - one or more coliform bacteria which, when grown on some media, form one distinct coliform colony.
Media - a nutrient rich gel or liquid which serves as food and support for the bacteria.
Is quantitative (gives a number of coliform forming units from >1 to 200).
100 ml of water is aseptically passed through a membrane filter which is then placed on a particular media which will "grow" green sheen colonies indicating coliform bacteria are present. Incubate the endo-agar plate with filter on it at 35 degrees C +/- 0.5 degrees C for 24 hours. Observe media plate and count the number of green sheen colonies seen. Then, a confirmation test is done by inoculating 3 different medias. This is done by swabbing all the colonies and dipping the swab into 3 different test tubes. The presence of gas bubbles after 24 hours at 35 degrees C +/- 0.5 degrees C indicates (confirms) presence of total coliform. One test tube containing "MUG" is incubated at 44.5 degrees C in a water bath for 24 hours. If the "MUG" test tube fluoresces under UV light then E. coli is present.
Other (non-coliform) bacteria on the filter, as well as dirt, iron, organic matter, etc.
Can take up to 3 days to confirm presence or absence of coliform in the sample. Interferences give inconclusive results. Retest with a method which is not as sensitive to the interfering contaminants.
Most probable number. quantitative.
Is qualitative (presence or absence) and is sensitive enough to detect one coliform forming unit in a 100 ml sample of water.
2.6 grams of prepacked medium is added to 100 ml of drinking water collected in a pretreated sterilized container. The container is shaken and then incubated for at least 28 hours at 35 degrees C +/- 0.5 degrees C.
After the 28 hours, if the sample is yellow, then it is negative. The test is complete.
If the sample is distinctly red or magenta, then the result is Positive for total coliform. Place the bottle under UV light If, under UV, there is fluorescence then E. coli is present.
None from turbidity or non-coliform bacteria.
Use #1 (free) chlorine DPD tablets on drinking water. Follow the manufacturer's directions from your test kit.
Turbitidy is a property which causes light to be scattered and/or absorbed rather than being transmitted through, in this case, a body of water in a straight line. It is measured as NTU's on a machine.
Organic matter, suspended matter like clay, dirt, sand, carbon (from filters), etc.
Nobody likes "dirty" water. Turbidity interferes with UV disinfection by blocking the light. Turbidity uses up free chlorine residual.
Measures intensity of light scattered at a 90 degree angle to the path of incident light. (From 0.02 to 800 NTU range.) Must check efficiency of machine on a daily basis using known standards. Calibrate the turbidimeter with standards in the same range as your sample.
certification requirements
Hudson Valley Regional Poison Control Center Phelps Memorial Hospital Center 701 North Broadway, North Tarrytown 10591 800-336-6997 914-366-3030
NYSDOH Radon Program: 800-458-1158
NYSDOH Community Sanitation and Food Protection: 518-402-7600
NYCDEP Shokan: 845-657-6972. Valhalla (Dutchess County Plan Review): Ed Polese 914-742-2069
NYSDEC New Paltz: 845-256-3000; Online databases; Spill Hotline 800-457-7362
Environmental Facilities Corp. (loans, grant administration): 800-882-9721
OSHA: 518-472-6085
Water supply related organizations and phone numbers
* AWWA Small System Hotline: 800-366-0107
* NY Section AWWA: 315-455-2614, POB 9 Syracuse 13211
* USEPA. Drinking Water Hotline: 800-426-4791, Region 2: 212-264-1800
* New York City Department of Environmental Protection
* National Drinking Water Clearinghouse at the National Small Flows Clearing House: 800-624-8301
* National Rural Water Association: 405-252-0629 PO Box 1428 Duncan, OK 73534
* NY Rural Water Association: 518-828-3155 fax: 518-828-0582 PO Box 487, Rte 23B Claverack 12513
* Rural Community Assistance Corporation: 703-771-8636
* NYSDOH Bureau of Public Water Supply Protection: 518-402-7650
* USGS. New York District: 518-431-0137, NY water quality data
* Chemtrec (emergency number for incidents): 800-424-9300
* NSF's list of approved products
* Public Service Commission: 800-342-3377
* National Safety Council: 800-621-7615
* Before you dig: Underground Facilities Protective Organization 800-962-7962 or 1-800-DontDig
* Burials: New York State Department of State Division of Cemeteries 518-474-6226
Low interest loans for water supply improvements are available from New York State. Contact the health department to see about eligibility requirements and application forms.
i K. L. Rakness, L. D. DeMers, B. D. Blank, Opflow, V22#7 July 1996
ii 2001 OSHA Industry report, quoted from Del Williams, Water Engineering and Management, March 2002